How can quat binding & cleaning techniques impact the effectiveness of your purchase of wholesale microfiber from Monarch Brands.
The rapid spread of COVID-19 has brought home the impact of a world that’s getting smaller every day. In light of the current situation, I thought it would be timely to discuss using the proper tools and basic techniques when disinfecting surface areas and removing microorganisms.
There are three significant categories of wipers. Monarch Brands Sells both natural cotton rags and synthetic microfiber products. We believe that when it comes to the appropriate tool, there is not a better product than microfiber.
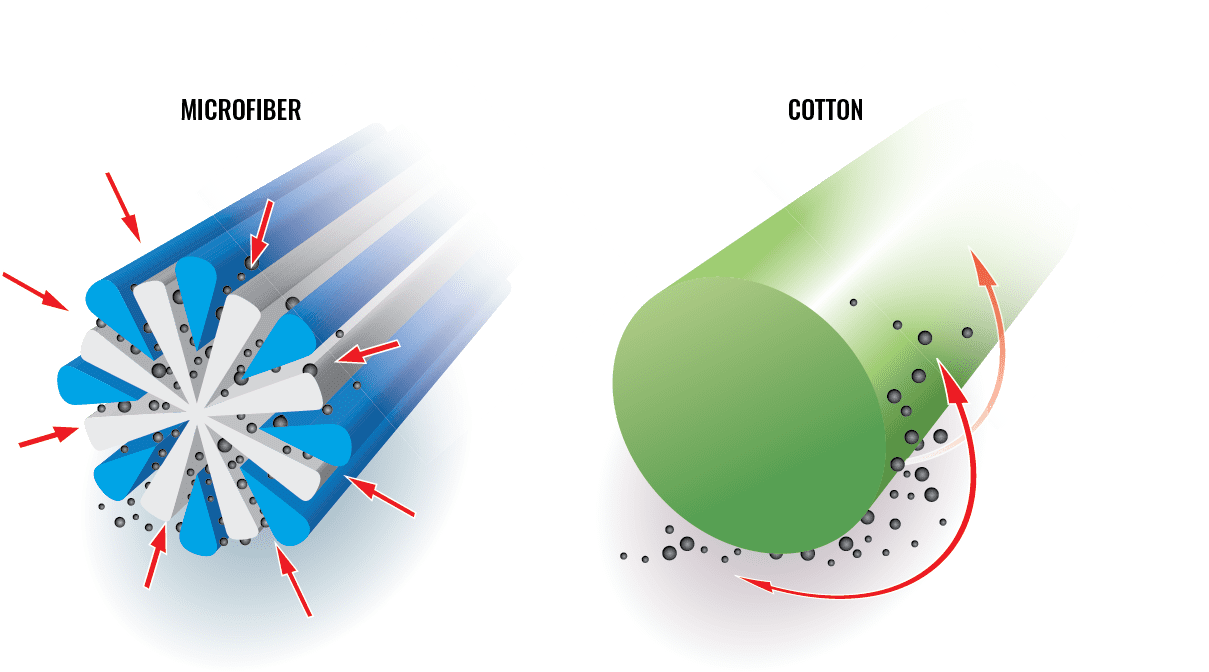
Cotton wiping cloths (rags) and string mops push dirt and bacteria around while removing very little when compared to microfiber products. Their natural fibers hold liquid but harbor bacteria, so they are usually good for a wipe and toss. Cotton rags are inexpensive but bulky. Rags excel in low-risk, high-loss environments as well as various finishing/staining applications.
Paper-based disposable products, by their very nature, do not cross-contaminate surfaces. Typical Paper products typically do not hold up to modest cleaning applications and lack the absorption and scrubbing power of microfiber cloths. There are robust non-woven products designed for hospital use (i.e., one cloth, one room, one application). These products are expensive and damaging to the environment.
Your cleaning technique plays a pivotal role in the sterility of the final result. Quat binding occurs when the active ingredient (quaternary ammonium chloride) becomes drawn to and incorporated into your cleaning textile.
The science behind how this happens is ‘elementary’: Quats contain positively charged ions, and cotton and other natural textiles contain a negative charge; positive attracts negative. Microfiber also includes a positive charge. However, the construction of microfiber (explained above) means that more liquid becomes trapped within the cloth. The result is that at least a portion of the quat does not end up on the surface it is supposed to be cleaning.
One study conducted by CleanLink found that the quat level of a disinfectant remaining on a cotton cloth placed in a solution-filled pail was decreased by 50 percent after soaking for just 10 minutes. That means the solution applied to the surface would contain only half of the parts per million (ppm) listed on the label. In another article on the subject CleanLink experts go on to state: “Microfiber is a better cleaning tool than cotton,” says Hicks. “But not all microfiber is created equal, so you need to do your research. If you continue to use quats, buy microfiber that doesn’t have cotton in it. Microfiber is also more effective at soil removal, and soil will deplete the active ingredient [in disinfectants].”
Like Hicks, Wilcox favors microfiber to prevent quat binding and remove soil and pathogens during the disinfection process. She also highlights its sustainability benefits.



 Author: David Forssell
Author: David Forssell



